Case report — Congenital abnormality of calves
Thomas Teoh (Senior veterinary officer – Epidemiology)
Jeff Cave (DVO Wodonga)
Karen Moore (Senior surveillance officer)
Jaimie Hunnam (Principal veterinary officer – Epidemiology)
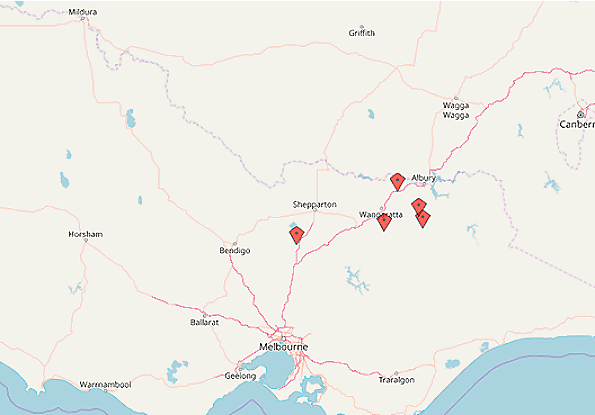
Between December 2017 and April 2018, there have been five investigations into cattle abortion where an arboviral aetiology has been suspected (Figure 1).
Among these, orthobunyaviruses such as Akabane virus, Aino virus and Schmallenberg virus, are important differentials to consider, and members of this group have been previously implicated in causing reproductive failures in cattle in parts of Australia and Japan.
One of the investigated properties from northern Victoria reported six abortions or postnatal deaths from a group of 65 maiden Angus cross shorthorn heifers. Approximately six of the heifers that had calved had dystocia issues.
Calves were born with fused joints, back legs shorter than the front legs, and flexed necks that could not be straightened (Figure 2). Blood samples were forwarded to the DEDJTR veterinary laboratory AgriBio for Akabane virus, Aino virus and Schmallenberg virus serology.
Virus neutralisation yielded a weak positive response to Aino virus within two of the animals, suggestive of a prior exposure. Aino virus is a Simbu serogroup orthobunyavirus, capable of manifesting reproductive defects in cattle leading to production losses.
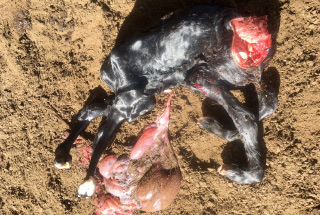
Clinical signs that have been associated with infection include arthrogryposis, cerebellar hypoplasia and hydrancephalus thought to be as a result of viral polymyositis, encephalomyelitis proceeding to necrosis of nervous tissue and atrophy of muscle.
A second property reported 12 of 20 heifers that had calved had dystocia issues. Calves were born alive but displayed signs of congenital arthrogryposis – stiff contracted limbs and ventroflexion of the neck. Calves typically died within 48 hours of birth. Differential diagnoses included Akabane virus, Aino Virus and Bovine Virus Diarrhoea (BVD).
Blood samples from one of the heifers and fresh and fixed samples from one of the calves were forwarded to AgriBio for testing. The heifer was negative for Aino and Akabane, but had a weakly positive reaction to the pestivirus AGID. Thoracic fluid from the calf was negative for Akabane virus, Aino virus and BVD.
Typically in cases such as these, accurately determining when cattle may have been exposed is challenging. An infection producing a deformed foetus towards a latter stage of gestation requires for the dam to be infected when the foetus is suitably developed. Too early and infection will result in early foetal death and a silent abortion.
While the clinical presentation in these cases resembles the effects of an Aino virus infection, without conclusive evidence of infection and a record showing a plausible chain of events demonstrative of cause and effect, these cases of abortion cannot be definitively attributed.
A case of Aino virus infection can be demonstrated using molecular diagnostic techniques for viral RNA segments or viral culture, from infected blood or tissue of the dam or foetus. Alternatively, anti-Aino virus antibodies within the foetal pre-colostral sera would indicate that the calf likely succumbed to a previous exposure to Aino virus.
The recommended specimens to collect when there are calf deformities are:
- maternal clotted blood
- specimens from aborted or deformed calves. These include fresh (chilled) cerebrum (1 to 2 cm maximum dimension), spleen, lung, heart blood or thoracic fluid, placenta and fixed brain, cervical spinal cord, lung, heart, liver, kidney, spleen, placenta.
References
Kitano Y, Ohzono H, Tsutomu S. Proliferation and Teratogenicity of Aino Virus in Chick Embryos. Microbiology and Immunology 1996;40:85–88.
Ishibashi K, Tomishita Y, Shirakawa H et al. Congenital Scoliosis of Calves Suspected of Aino Virus Infection. Journal of the Japan Veterinary Medical Association 1994;47:87–90.