Soil acidity
Soil acidity is a potentially serious land degradation issue. When soil becomes too acidic it can:
- decrease the availability of essential nutrients
- increase the impact of toxic elements
- decrease plant production and water use
- affect essential soil biological functions like nitrogen fixation
- make soil more vulnerable to soil structure decline and erosion.
Without treatment, soil acidification can impact agricultural productivity and sustainable farming systems. Acidification can also extend into subsoil layers, posing serious problems for plant root development and remedial action.
What causes soil acidity?
Soil acidification is a natural process, but it can be increased by some agricultural practices.
Acidification occurs in agricultural soils as a result of the:
- removal of plant and animal products
- leaching of excess nitrate
- addition of some nitrogen based fertilisers
- build-up in mostly plant-based organic matter.
Soil acidity occurs naturally in higher rainfall areas and can vary according to:
- the landscape geology
- clay mineralogy
- soil texture
- buffering capacity.
How acidity affects plant growth
Acidity itself is not responsible for restricting plant growth. Instead, biological processes favourable to plant growth can be negatively affected by acidity.
Acidity has the following effects on soil:
- It decreases the availability of plant nutrients, such as phosphorus and molybdenum, and increases the availability of some elements to toxic levels, particularly aluminium and manganese.
- Essential plant nutrients can also be leached below the rooting zone.
- Acidity can degrade the favorable environment for bacteria, earthworms and other soil organisms.
- Highly acidic soils can inhibit the survival of useful bacteria, such as the rhizobia bacteria that fix nitrogen for legumes.
Soil pH as a measure of acidity
Soil pH is a measure of acidity or alkalinity. A pH of 7 is neutral, above 7 is alkaline and below 7 is acid. Because pH is measured on a logarithmic scale, a pH of 6 is 10 times more acid than a pH of 7.
Soil pH can be measured either in water (pHw) or in calcium chloride (pHCa) and the pH will vary depending on the method used. As a general rule, pH measured in calcium chloride is 0.7 of a pH unit lower than pH measured in water.
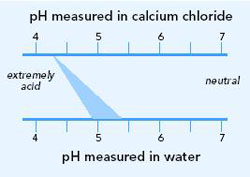
There are several differences between pHCa and pHw:
- Soil pHCa measurements in Australia vary from pHCa 3.6 to pHCa 8 for a range of different soil textures (sandy loams to heavy clays). Soil pHw values lie between pHw 4 and pHw 9.
- The pHw may be higher by 0.6 to 1.2 in low salinity soils and higher by 0.1 to 0.5 in high salinity soils. Research has shown a difference of 0.7 for a wide range of soils.
- Higher pHw values to around 10 may be associated with alkali mineral soils containing sodium carbonates and bicarbonates.
- Research has shown that seasonal variation of pHw can vary up to 0.6 of a pH unit in any one year. In comparison, soil pHCa measurements are less affected by seasons.
When a laboratory measures your soil's pH, make sure they specify which method (water or calcium chloride) was used.
Soil pH levels
A pHCa range between 5 and 6 is considered ideal for most plants. Acid soils have a major effect on plant productivity once the soil pHCa falls below 5:
- pH 6.5 — close to neutral — Optimum for many acid-sensitive plants. Some trace elements may become unavailable.
- pH 5.5 — slightly acid — Optimal balance of major nutrients and trace elements available for plant uptake.
- pH 5.0 — moderately acid — Below pH 4.8 aluminium (Al) can become toxic to plants, depending on soil type. Phosphorus combines with Al and may be less available to plants.
- pH 4.5 — strongly acid — Aluminium becomes soluble in toxic quantities. Manganese (Mn) becomes soluble and toxic to plants in some soils, depending on temperature and moisture conditions. Molybdenum (Mo) is less available. Soil bacterial activity is slowed down.
- pH 4.0 — extremely acid — Irreversible soil structural breakdown can occur.
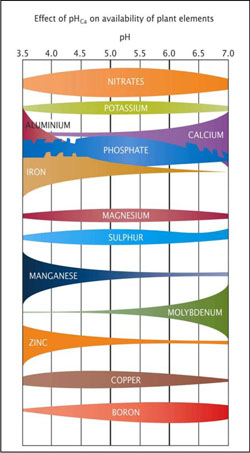
Soil pH will influence both the availability of soil nutrients to plants and how the nutrients react with each other.
For example:
- At a low pH, many elements become less available to plants, while others such as iron, aluminium and manganese become toxic to plants. Aluminium, iron and phosphorus also combine to form insoluble compounds.
- At a high pH, calcium ties up phosphorus, making it unavailable to plants, and molybdenum becomes toxic in some soils. Boron may also be toxic in some soils.
Testing soil pH
Soil pH is one of the most routinely measured soil parameters. This is because:
- testing is relatively easy
- field equipment to measure pH is relatively inexpensive.
Don't rely on field test kits for decisions such as rates of lime application. Test kits will only tell you whether your soil is acid or alkaline. You're unlikely to get responses to lime if other nutrients are lacking.
Professional soil sample analysis by a recognised laboratory will ensure the most accurate results.
Liming to correct soil pH
For most acid soils, the most practical management option is to add lime to maintain the current soil pH status or increase surface soil pH.
For a better chance at successfully growing acid-sensitive species, consider liming once the pH drops below pHCa 5.0.
If paddocks with an acidity problem are not limed, the soil pH will continue to fall and settle at pHCa 3.8 to 4.2.
Lime application for permanent pasture
In permanent pasture situations, spreading the lime on the surface and allowing it to work its way into the soil is acceptable. Surface application is better than no application.
Lime responses are generally seen in the first and second year for cropping systems, but can take up to 5 years depending on soil type, rainfall and lime quality for permanent pasture systems.