Canola diseases
Canola can be infected by a number of pathogens in Australia ranging from root rots to leaf disease and crown to stem infections.
As with all diseases their presence and severity is dependant on plant susceptibility, presence of the pathogen and favourable climatic conditions.
Generally fungal disease such as blackleg and Sclerotinia are more damaging in higher rainfall regions but if unseasonably high rainfall occurs in lower rainfall regions these areas may also experience high disease levels.
Disease control varies for each pathogen but generally variety resistance, crop production practices and fungicides are used either in isolation or combination to reduce economic losses.
If growers are aware of the disease risks in their area and follow strategic management plans they should be able to adequately control most canola diseases.
Blackleg
Blackleg, caused by the fungus Leptosphaeria maculan, is the most damaging disease of canola (Brassica napus) in Australia and most canola producing countries throughout the world.
Sclerotinia stem rot
Sclerotinia stem rot, caused by the fungus Sclerotinia sclerotiorum, is a disease that attacks many species of broadleaf plants, including:
- canola
- peas
- beans
- sunflowers
- lupins.
The disease is sporadic, occurring when environmental conditions are favourable for infection. Prolonged humid (wet) conditions during the flowering period favour disease development and yield losses as high as 24 per cent have been recorded in Australia.
Symptoms
The disease infects canola crops from late flowering onwards, with symptoms appearing two to three weeks after infection.
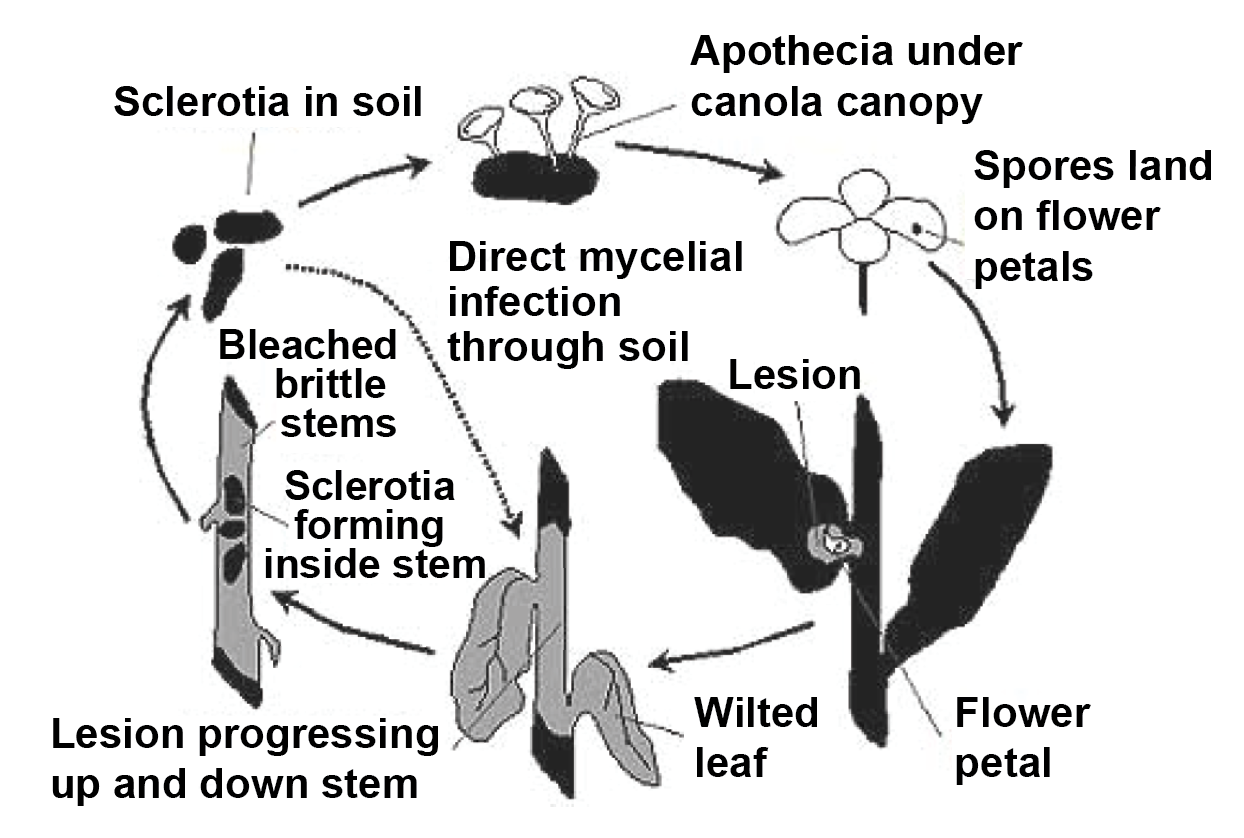
The fungus produces light brown discoloured patches on stems, branches and pods. These lesions expand and take on a greyish-white colour. Infected canola plants ripen earlier and stand out as bleached or greyish coloured plants among green healthy plants.
The bleached stems tend to break and shred at the base. When an infected canola stem is split open, hard black bodies called sclerotia can usually be found inside. Sclerotia are the resting stage of the fungus and resemble rat droppings (dark coloured structures either round like canola seed, rod, cylindrical or irregular in shape, 2 to 4 mm in diameter and up to 20 mm long).
In wet or humid weather, a white growth resembling cotton wool can develop on lesions and sclerotia may also develop in this white growth.
Disease cycle
Sclerotia remain viable for many years in the soil.
When weather conditions are favourable, the sclerotia germinate to produce small mushroom-shaped structures called apothecia. Apothecia produce thousands of air-borne spores that can be carried several kilometres by the wind.
Spores land on canola petals, germinate, and then use the petal as a nutrient source producing a fungal mycelium. When the petals fall at the end of flowering, they often get caught in the lower canopy of the crop allowing the fungus to grow from the petal into the plant.
The canola flowering period is therefore the critical time for Sclerotinia infection. Germination of the spores and infection is enhanced by wet weather at flowering.
Management
- Yield losses in overseas crops can be reduced by the timely application of fungicides during flowering.
- Use good quality seed that is free of sclerotia. Although there is no canola seed certification for Sclerotinia in Australia, careful inspection of seed before sowing will determine if high levels of sclerotia are present.
- Sclerotinia does not affect cereals and sclerotia density will decline in the absence of a host. A 3 to 4 year break between canola crops and other susceptible crops reduces disease severity.
- Control broad leaf weeds to prevent build-up of inoculum.
- Avoid sowing canola adjacent to paddocks that were severely infected with Sclerotinia in the previous season.
Alternaria leaf and pod spot (black spot, dark leaf spot, Alternaria blight)
Alternaria is usually caused by the fungal pathogen Alternaria brassicae and occasionally by Alternaria brasicicola. Canola cultivars are more resistant to A.brassicicola.
The severity of the disease varies between years and locations depending on seasonal conditions. The disease is favoured by warm humid conditions during spring. Yield loss is unusual and is normally associated with the shattering of infected pods.
If infected seed is sown, seedling blight may occur (refer to damping-off section).
Symptoms
Alternaria infects all growth stages of canola plants. However, as plants mature from mid flowering onwards they are more susceptible to infection.
Alternaria symptoms can be found on all parts of the plant including leaves, stems and pods. Spots on leaves and pods have a concentric or target-like appearance and are brown, black or greyish white with a dark boarder. Lesions on green leaves are often surrounded by a chlorotic (yellow) halo.
Severe pod infections may cause seed to shrivel and the pods to prematurely ripen and shatter. Stem spots are elongated and almost black. Pod symptoms of Alternaria are similar to those of blackleg and the two can be difficult to distinguish in the field.
Disease cycle
Alternaria spp. survive the intercropping period on infected canola stubble, on cruciferous weeds and to a lesser extent on seed.
Seed infections can cause seedlings to rot (refer to damping-off section) resulting in a seedling blight that reduces plant establishment.
Initial crop infections are caused by wind blown spores. Spores remain intact on susceptible plants until moisture from dew or rain allows them to penetrate into the tissue and cause a lesion. These lesions produce further spores and infections can then be spread throughout the crop by either the wind or rain.
Mild, humid conditions favour disease development and the disease cycle will continue throughout the season under favourable conditions. Hot and dry conditions interrupt epidemics as the absence of moisture greatly reduces spore production.
Major outbreaks are not common in Australia as weather conditions are normally hot and dry throughout podding which is unfavourable for prolonged infection.
Management
Alternaria is very common in canola crops but is not usually severe enough to warrant control. In Australia, there are no registered fungicide seed treatments for Alternaria.
If pods were infected in the previous season, obtain fresh disease free seed.
In areas where Alternaria is a problem, select paddocks isolated from last years canola stubble as Alternaria spores are easily transported by wind and can spread into areas that have not had canola for several years.
Clubroot in canola and Juncea canola
Clubroot is caused by the soil-borne fungus Plasmodiophora brassicae.
The disease occurs worldwide and only affects plants in the Cruciferae family including:
- canola
- juncea canola (mustard)
- cabbage
- cauliflower
- brussels sprouts
- broccoli.
In Australian vegetable Brassicas, clubroot is widespread and causes significant yield losses.
The Australian oilseed industry has been somewhat protected from clubroot as the major production areas for vegetable and oilseed brassicas are usually separated from one another.
In addition, most Australian pathotypes of clubroot are only able to cause disease in the warmer months and require irrigation water for dispersal, with the exception of Tasmania and some parts of New South Wales where disease is observed year round.
Symptoms
Swollen, galled roots are the most typical symptom of infected plants.

This ranges from tiny nodules, to large, club-shaped outgrowths. The galls are at first firm and white but become soft and greyish brown as they mature and decay. Affected roots have an impaired ability to transport water and nutrients.
Disease Cycle
Resting spores of the fungus can survive in soil for many years, even in the absence of a susceptible host.
Infection can occur at any stage of growth and is restricted to the roots. In the presence of susceptible roots, the spores germinate and release tiny motile spores that swim in free water to the surface of the rootlets, penetrate and form a fungal colony (plasmodium) inside the root cells.
The fungus causes cells to enlarge and divide rapidly, resulting in the characteristic galls.
Late in the season, resting spores develop in the infected roots and are released into the soil as the galls decay. Fields become infested mainly by the movement of soil on cultivation equipment and by seedling transplants.
Management
In the Australian vegetable brassica industry several methods of control have been developed that may be useful for oilseed brassicas:
- 5 year rotation: infested fields are kept free of susceptible crops and weeds for at least 5 years, to allow sufficient natural decay of the long-lived spores
- equipment movement: do not move cultivating equipment from infested to non-infested areas before thoroughly cleaning the equipment
- liming: clubroot thrives in acid soils (pH <7.0), liming to increase soil pH (7.0-7.5) has been successful for vegetable brassicas but would be cost prohibitive in most oilseed brassica areas.
Damping-off (seedling blights and seedling hypocotyl rot)
Damping-off is usually caused by the fungus Rhizoctonia solani. However, other fungi including Fusarium spp., Pythium spp., Phytophthora spp., and Alternaria spp. and the blackleg fungus, Leptosphaeria maculans, can also cause damping-off.
Symptoms and crop management are similar for all these pathogens so they are grouped together and referred to as 'damping-off'.
All species are common inhabitants of the soil and cause damage when conditions are not ideal for early seedling growth. Problems are usually seen when seed is sown dry, close to the autumn break (within a couple of weeks of a normal break) or if weather conditions become cool and damp.
Yield loss is unusual unless plant numbers are severely reduced or patchy establishment occurs.
Symptoms
Damping-off can produce many symptoms ranging from pre-emergence rot (failure of plants to emerge) to post-emergence damping-off (plants emerge and collapse at ground level).
If affected plants survive, they are normally stunted and may flower and mature prematurely. Once past the seedling stage canola plants are not adversely affected by damping-off.
Both pre and post-emergent damping-off occur in patches and affected areas can spread quickly during cold wet conditions. Leaves of plants affected by post-emergent damping-off may become discoloured, turning orange, purple and / or chlorotic. In some cases, the tap root is dark in colour and shrivelled at ground level.
These symptoms should not be confused with insect damage where root or stem tissue has been removed.
Disease cycle
Damping-off fungi are soil-borne and survive in the soil by forming resistant resting structures when no host is present. These resting structures germinate with the break of the season and the fungi grow through the soil until they find a susceptible host plant.
Dry seeds become vulnerable to attack as soon as they begin to germinate. Once in the plant, the fungi multiply causing decay that damages or kills the seedling. Damping-off fungi are usually weak pathogens (except blackleg) only able to infect young succulent tissue.
At the two to four leaf stage, below ground parts of canola plants become woody enough to withstand further infections. Therefore, most damage occurs when wet and cold weather slows plant growth.
Temperature and soil moisture affect disease development. Loose, cold and dry soils favour Rhizoctonia solani, while cold damp soils favour Fusarium spp. and wet, heavy soils favour Pythium spp.
Management
- Yields are only affected when plant numbers are severely reduced. If seedling loss is uniform throughout the crop, surrounding plants can often compensate by growing larger. If seedling loss is patchy and large areas die then re-sowing may be required.
- Damping-off fungi will germinate with the opening rains of the season. Once they have germinated they are very successfully controlled by soil tillage. Therefore dry-sown or crops sown very close to the opening rains may be more severely affected. If crops are re-sown the sowing tillage will generally control the fungi.
- Application of a seed fungicide treatment at sowing can reduce damping-off damage.
Downy mildew
Downy mildew is a common disease of canola throughout the world, and is caused by the fungus Peronospora parasitica.
Infection occurs under cool moist conditions where leaves or cotyledons are in contact with the soil or other leaves. Although seedlings can be severely attacked by the disease, significant yield loss does not usually occur. Downy mildew is rarely found beyond the rosette stage and crops normally grow away from it with the onset of warmer weather.
Symptoms
Chlorotic or yellow areas on the upper leaf surface are the first symptoms to occur. These can be seen on young seedlings when cotyledons or first true leaves are present.
A white mealy fungal growth can be seen on the underside of the leaf beneath these spots, if conditions have been moist or if dew periods have been long. Infected cotyledons tend to die prematurely.
As the disease develops, individual spots join to form large irregularly shaped blotches. These necrotic lesions may cause a large part of the leaf to dry out and the upper surface of the leaf to develop a yellow red colour.
Disease cycle
The fungus is both soil and seed-borne and can persist in the soil for a long time.
Infection is favoured by cool wet weather and under ideal conditions, new infections can develop in as little as 3 to 4 days. The fungus is related to white rust with specialised spores (oospores) probably responsible for primary infections.
Conidial spores produced on the underside of the infected leaf are then responsible for the secondary spread of the disease.
Management
- Downy mildew does not usually affect yield so control measures are not generally warranted unless plant densities are severely reduced on a regular basis.
- In areas where downy mildew is a severe problem fungicides containing copper as the active ingredient are registered for use in Australia.
- Crop rotation and the control of cruciferous weeds between canola crops can reduce disease severity.
Viruses
There are three viruses that infect Australian canola:
- Turnip yellows virus (TuYV) formerly known as Beet western yellows virus (BWYV)
- Cauliflower mosaic virus (CaMV)
- Turnip mosaic virus (TuMV).
These viruses are widespread and surveys have found that in some situations most crops have some infected plants. As with most viruses, yield loss is very hard to measure, so the actual damage to crop production is difficult to determine.
Symptoms
Symptoms are very variable, from no visual indication, to stunted red plants and stiffening of leaves for TuYV, chlorotic ring spots and mottling for CaMV and yellow mosaic patterning and tip necrosis for TuMV.
Disease cycle
These viruses are not seed-borne. They survive in weeds or volunteer host plants during the summer and are then spread from these plants into crops by aphids which act as the vector for transmission of these viruses.
TuYV is termed a persistent virus. Persistent viruses are carried in the aphid's body and can be transmitted to healthy plants during feeding. The aphid will often remain infective throughout their life.
CaMV and TuMV are non-persistent viruses being retained in the aphid mouthparts for less than 4 hours.
Autumn is the critical infection period, so the earliest-sown crops usually have the highest infection incidence. Yield loss is greater in crops that have been infected as seedlings. Infections can occur past the rosette stage of canola growth but these probably have little effect on yield.
Management
- Control broadleaf weeds (especially over summer) as they can act as reservoirs for the viruses.
- Sow at recommended times - earlier sown crops usually have a greater incidence of viral infection.
- Monitor aphid numbers in crops (aphids usually found under leaves) and consider using an insecticide as a foliar (active ingredient primicarb) or seed treatment (active ingredient imidacloprid) to control aphids on seedlings and young canola plants.
White leaf spot
White leaf spot is caused by the fungus Mycosphaerella capsellae (also called Pseudocercosporella capsellae). The disease has a worldwide distribution and a wide host range among cruciferous weeds. In Australia, white leaf spot commonly infects canola seedlings. It is not usually severe enough to cause yield loss.
Symptoms
Leaf, stem and pod lesions are greyish-white to light brown. Unlike blackleg lesions, white leaf spot lesions do not contain pycnidial fruiting bodies (black dots) and usually have a more granular surface compared with the smooth surface of blackleg lesions.
Leaf lesions often have a brown margin when they mature. They can be up to one centimetre in diameter and often join to form large irregular shaped lesions. Nutrient deficient crops have been reported to be more severely affected by the disease.
In severe epidemics, infections can defoliate susceptible varieties

Disease cycle
The fungus survives on canola stubble as thick-walled mycelium.
When prolonged wet weather conditions prevail during autumn and winter, wind-borne spores are produced that cause primary leaf lesions on canola. These initial lesions go on to produce new wind-borne spores that cause the rapid spread of disease throughout the crop.
The disease is not usually seed-borne but can be spread by infected seeds or infected debris with the seed.
Management
White leaf spot infection is not usually severe enough to warrant control. It can be managed by:
- rotating crops and isolating from the previous year's canola stubble - this will prevent infection from wind-borne spores
- controlling cruciferous weeds and volunteer canola
- providing adequate nutrition to reduce crop stress.
White rust or Staghead
White rust is caused by the fungus Albugo candida.
The disease is uncommon on B. napus (Australian canola varieties) but does infect B. juncea (juncea canola / Indian mustard) and the weed shepherds purse (Capsella bursa-pastoris).
Symptoms
White to cream coloured pustules form on the underside of leaves and on floral parts. These pustules rupture the host epidermis exposing a white chalky dust. On the upper surface of the leaves, the infected areas are bleached and thickened.
Systemic infections of the growing tips and flower heads give rise to stagheads. Stagheads are very conspicuous in the crop as swollen, twisted and distorted flower heads that produce little to no seed and become brown and hard as they mature.
Symptoms for white rust should not be confused with severe calcium deficiency symptoms that cause flowering stalks to collapse resulting in the withering death of the flower head.
Disease cycle
Resting spores (oospores) of the fungus can survive in infected plant material or as a seed contaminant for many years when conditions remain dry.
When conditions become moist the resting spores are able to directly infect plants. However, they usually produce tiny motile spores that can swim in free water to infect seedlings, causing cream-white pustules to form.
Inside the pustules new swimming spores are formed and then distributed throughout the canopy by rain splash to form secondary infections. They do this by growing through stomata into adjacent cells, causing systemic infections and subsequent stagheads if the growing tips of plants become infected.
The resting spores can be formed in any infected tissues but are present in larger numbers in stagheads. When the crop is harvested, stagheads break releasing resting spores that contaminate harvested seed or blow out to contaminate the soil.
Management
- obtain seed from disease free or low disease crops
- control cruciferous weeds
- extended rotations will allow crop residues to decompose and reduce the risk of infections
- if warranted consider growing B. napus rather than B. juncea.
Contact
Field Crops Pathology
Grains Innovation Park
110 Natimuk Rd
Horsham 3400
03 5450 8301
Or call the Customer Service Centre, 136 186
Acknowledgements
Steve Marcroft, Marcroft Grains Pathology, Chris Bluett, Tamrika Hind-Lanoislet, NSW-DPI.